Somatosensory Evoked Potentials (SSEP)
Somatosensory evoked potentials (SSEP) are recorded from the central nervous system following stimulation of peripheral nerves.
Introduction
Somatosensory Evoked Potentials (SSEPs) are electric signals recorded from the scalp or spine following stimulation to the peripheral nerves. They are time-locked responses, representing the function of the ascending sensory pathways. Early in the 1960s Larson et al introduced the use of somatosensory evoked potentials to monitor neural structure during neurosurgical procedures. It was utilized as a supplement to the wake-up test during correctional spinal surgeries for spinal deformities such as scoliosis to provide warning of compromised spinal cord function to the spine surgeons, as reported by McCallum et al and Nash et al in the 1970s. Since then SSEP has become one of the earliest and primary tools for intraoperative neurophysiological monitoring.
Somatosensory Pathways
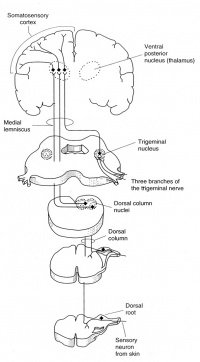
Distal peripheral nerves are stimulated for SSEP recordings; typically the median or ulnar nerve at the wrist for upper extremity SSEPs, or posterior tibial nerve at the ankle for lower extremity SSEPs. The ascending sensory volley enters the spinal cord through dorsal nerve roots. Multiple dorsal or posterior column spinal pathways such as the gracile fasciculus (legs and trunk) and the cuneate fasciculus (arms and trunk) mediate the SSEP responses. They arrive at the medulla and make synaptic connection at the medullary nuclei (nucleus cuneatus and nucleus gracilis). From there they cross and ascend in the medial lemniscal pathways to thalamic nuclei, which in turn project up to the somatosenory cortex.
There is no synapses between the peripheral stimulation sites and the medullary nuclei. Synapses are the sites of action for inhalational anesthetic agents. Therefore any SSEP responses recorded below the level of medullary nuclei are affected only minimally by general anesthesia. The cortical SSEP responses, however, are greatly affected by inhalational agents; whichever recorded anesthetic management is very much required.
Stimulation
- Stimulation Sites: SSEPs may be elicited by electrical stimulation to major nerve trunks or dermatomes. Upper extremity mixed or major nerve SSEPs are typically obtained by stimulating the median nerve or ulnar nerve near the wrist; or sometimes the ulnar nerve at the elbow. Lower extremity SSEPs are normally recorded to stimulation of the posterior tibial nerve at the ankle, or peroneal nerve near the head of the fibula at the knee. Usually the anode electrode should be placed 2-4 cm distal to the cathode electrode to avoid anodal block.
- Electrodes: Subdermal needle electrodes or adhesive surface electrodes are most commonly used for intraoperative SSEP recordings. Some other types of electrodes such as bar electrodes and EEG metal disc electrodes are more suitable for diagnostic SSEP recordings in the lab. Needle electrodes can be placed closer to the underlying nerves than surface electrodes; however they are associated with additional risks of infection, bleeding, and inadvertent needle sticks.
- Constant Current vs Constant Voltage: Constant current stimulation is recommended for consistent and reliable stimulation for optimal SSEP recording in the OR, especially for long surgical procedures. Constant current stimuli may compensate for any changes in electrical conductivity, or electrode contact resistance.
- Stimulation Parameters: a series of rectangular pulses with certain pulse width and frequency are typically used as electrical stimuli for SSEP recording.
- Pulse width (or pulse duration): 200-300 microsecond is suggested
- Frequency (or rep rate): Higher stimulus rate is desired for quick acquisition of SSEP responses in the operating room. However increasing the rate too high may result in degradation of the responses; also it is limited when interleaving stimulation of 2 or 4 limbs. In general a frequency between 2 and 5 Hz are recommended. To avoid synchronization between the responses and the underlying electrical noise, most commonly 60Hz or 50Hz in different countries, the stimulus rate should not be a submultiple of the noise frequency. Sometimes a slight change of stimulus rate, for example from 4.80 to 4.13, may improve the quality of the evoked responses.
- Intensity: Supramaximal stimulation should be used to produce repeatable responses. Some factors, such as pathology of the peripheral nerves, large or edematous extremities, distance of the electrodes to the underlying nerves, types of stimulating electrodes, may limit the effectiveness of stimulation. A stimulus of 50mA or greater is required sometimes. There is concern of tissue damage from high current densities at the stimulation site which is rare.
Recording Techniques
- Electrodes: Metal surface cup electrodes are non-invasive electrodes, which can be applied with conductive gel after skin preparation. Subdermal needles are also commonly used in the operating room; some are available with angled or hooked tips. A corkscrew version can be screwed into the scalp for prolonged recording, however excessive bleeding can be a concern. For direct cortical mapping and recording, a strip or grid electrode array can be used for cranial surgeries.
- Recording Sites
- Cortical recording of upper extremity SSEP: It is the post-central gyrus of somatosensory cortex, contralateral to the stimulated limb. The locations are called CP3 and CP4 which are 2 cm posterior to the C3 and C4 positions of the 10-20 International System of EEG electrode placement. The recording montage can be CP3-Fz or CP3-CP4 for right arm stimulation; CP4-Fz or CP4-CP3 for left arm stimulation.
- Cortical recording of lower extremity SSEP: CPz is the active electrode site, which is 2 cm posterior to Cz. The traditional derivation of recording is CPz-Fz; however it should not be the only standard recording derivation, according to studies for optimized lower extremity SSEP recording by MacDonald DB et al and many others. Often time CPz-CPc (which is CPz-CP4 for left leg stimulation, and CPz-CP3 for right) may produce higher amplitude and more reliable signals.
- Subcortical: The recording can be made at posterior cervical spine, one or linked earlobes, or mastoid. Subcortical responses are less affected by inhalational agents. However, the subcortical SSEP response is normally very well defined for upper extremity stimulation, it is often poorly defined for lower extremity stimulation.
- Peripheral nerve: Usually it is the ipsilateral Erb's point for upper extremity stimulation, and ipsilateral popliteal fossa for lower extremity stimulation. This is done to verify the status of the peripheral stimulation.
- Averaging
- Recording Parameters
- Filters
- Timebase
- Sensitivity
- Sweep delay
- Interleaving recording
Waveform
Intraoperative Monitoring
The basic principle of mixed nerve SSEP monitoring is to stimulate distal to the surgical site at risk and to record at a sites(s) proximal to the surgical site. In most cases, these recording sites should include at least one cortical and one subcortical recording site.
Anesthesia and Other Factors
References
- Larson SJ, Sances A. Evoked potentials in man: neurosurgical applications. Am J Surg 1966; 111: 857-861.
- MacDonald DB. Individually optimizing posterior tibial somatosensory evoked potential P37 scalp derivations for intraoperative monitoring. J Clin Neurophysiol. 2001 Jul;18(4):364-71.
- McCallum JE, Bennett MH. Electrophysiologic monitoring of spinal cord function during intraspinal surgery. Surg Forum 1975; 26: 469-471.
- Nash CL, Long RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop 1977; 126: 100-105.
- Powers SK, Bolger CA, Edwards MSB. Spinal cord pathways mediating somatosensory evoked potentials. J Neurosurg 1982; 57: 472-82.
- Simpson RK JR, Blackburn JG, Martin HF III, Katz S. Peripheral nerve fibers and spinal cord pathway contribution to the somatosensory evoked potentials. Exp Neurol 1981; 73: 700-15.
- Toleikis JR. Intraoperative monitoring using somatosensory evoked potentials: A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005; 19(3):241-58.
- Wang BP, Turner LA, Kamp AM, Venier LH. CPz-CP4 and CPz-CP3 are superior to CPz-FPz for recording left and right tibial nerve somatosensory evoked potentials for intraoperative monitoring. A review study in 264 patients. 2006 The 17th Annual Meeting of American Society of Neurophysiological Monitoring.